Contec Fingertip Pulse Oximeter with CE and FDA
Basic Info
| Model NO. | CMS50-PRO |
| Classification | Physiological Functions of Diagnosis and Monitoring Equipment |
| Type | Fingertip Pulse Oximeter |
| Certification | CE, FDA, ISO13485 |
| Group | Middle-aged and Old |
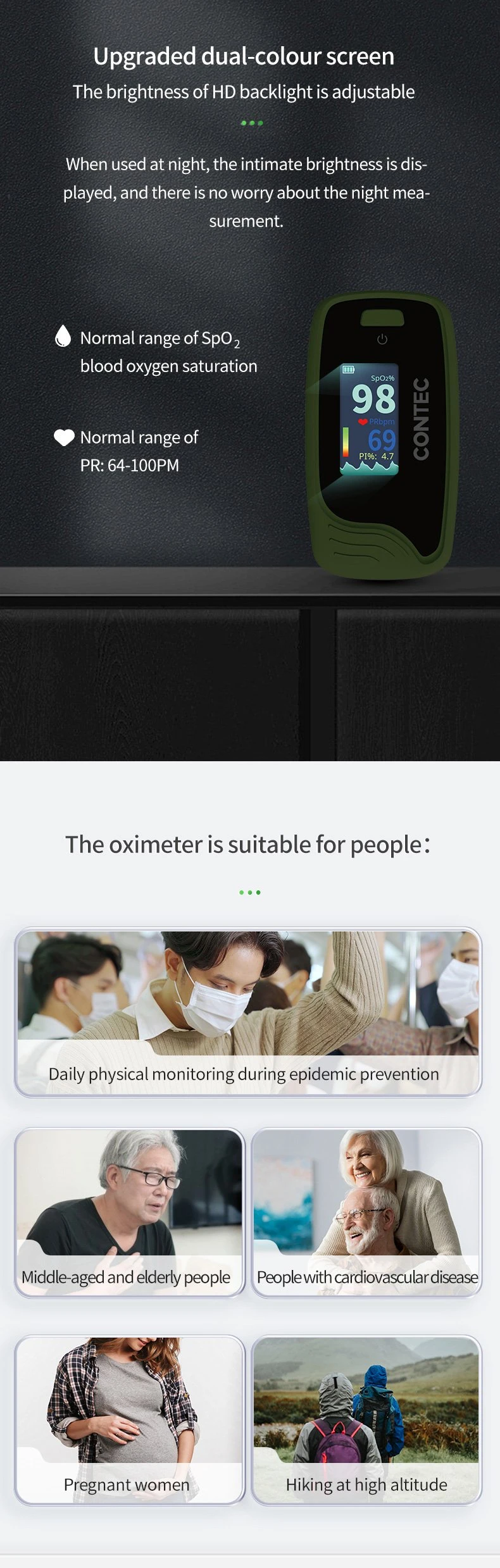
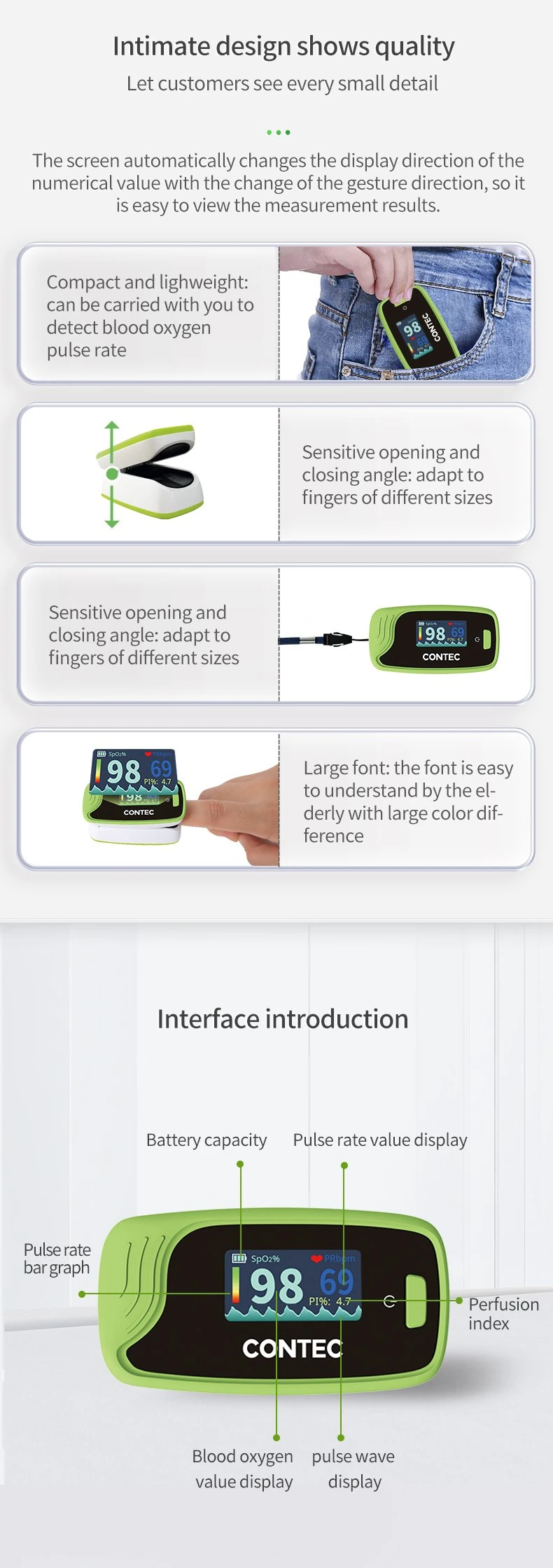
| Pulse Rate Value Display | Yes |
| Bar Graph Display | Yes |
| Pulse Waveform Display | Yes |
| Display Mode | Can Be Changed |
| OEM/ODM | Yes |
| Function | Fingertip Pulse Oximeter for Adult |
| Transport Package | Carton |
| Specification | 60(L) mm & 30.5(W) mm & 32.5 (H) mm |
| Trademark | CONTEC |
| Origin | China, China |
| HS Code | 9018909911 |
| Production Capacity | 10000/Week |
Product Description
CONTEC Fingertip Pulse Oximeter with CE and FDAProduct Description
Model:CMS50-pro
Product List
-TELEMEDICINE products-Pulse oximeter-Patient Monitoring-ECG machine(Electrocardiograph)/Holter ECG/Stress test ECG,-EEG,-Ultrasound Imaging,Pocket fetal doppler,fetal monitor,-Blood pressure Monitor(ABPM),-spirometer-infusion/syringe Pump-Digital Stethscope-In-Vitro Diagnostics-simulator-Veterinary products
View more products,click here...
Company Profile
| Business Type | Manufacturer, Trading Company | Country / Region | Hebei, China |
| Main Products | Pulse Oximeter, Pocket Fetal Doppler, Patient Monitor, ECG, Ultrasound Imaging | Ownership | Public Company |
| Total Employees | Above 1000 People | Total Annual Revenue | 2009943846 USD |
| Year Established | 1996 | Certifications(6) | ISO13485, FSC, ISO14001, Other, ISO9001, ISO13485 |
| Product Certifications(2) | CE, RoHS | Patents | Y |
| Trademarks | CONTEC | Main Markets | Domestic Market30.00%North America18.00%Western Europe15.00% |
Contec Medical Systems (hereinafter r is a high-tech company which focuses on research, manufacture and eferred to as CONTEC)distribution of medical devices since 1996, CONTEC locates in beautiful seaside city, Qinhuangdao, and is one of the largest bases for R&D and manufacturer of medical devices in China.In August 2020, CONTEC officially became a listed company in Shenzhen Stock Exchange (Stock Code:300869).CONTEC has more than 1,000 employees, covering an area of 67,450 square meters. we have a complete R&D, production and sales system, also we have got ISO9001, ISO13485 quality management system certification, ISO14001 environmental management system certification, BSCI Business Social Compliance Initiative and many other international standards Certification.The company has strong technical force and has obtained a number of core technology patents with independent intellectual property rights. Since its foundation, CONTEC is dedicated to develop and expanding our line of medical devices, till now we have more than 20 categories of products containing Pulse Oximeter, Sphygmomanometer, ECG, EEG, Ultrasound Equipment, Patient Monitor and Image Equipment, etc.,, and we own more than 100 national patent, 56 software copyright, our products have got CE, FDA and Canada/Japan/Brazil approval. Now, CONTEC products were distributed to over 130 countries and regions.People-oriented, innovative and win-win is CONTEC's development philosophy, CONTEC will take its advantage on technology and talent, furthermore promote its market internationalization, modern management, product diversification, and endeavors to build CONTEC a world-class enterprise and achieve sustainable & healthy development.